Dear Trice Distribution Team,
Get ready for the new EU Regulations:
On May 21, 2021 new Medical Devices Regulation (EU) 2017/745 (MDR) will bring EU legislation into line with technical advances, changes in medical science and progress in law-making.The new Regulations will create a robust, transparent, and sustainable regulatory framework, recognised internationally, that improves clinical safety and creates fair market access for manufacturers and healthcare professionals.
For our distributors in the EU, I will be holding an informational session to prepare you for the changes and ensure that we are all in compliance with the new MDR rules and regulations. Look for an invitation from me via email in the coming weeks.
What’s New:
Released on March 29th, 2021:
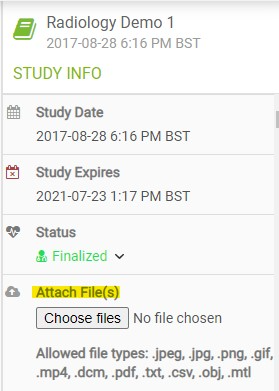
The study "Attach file" option will now allow files up to about 200 MB. You can find the "Attach file" option on the Side Information Panel under " Study Info".
 To keep up with the latest product updates and systemwide service notifications please sign up here: https://trice.statuscast.com
We Use Tricefy:
 This quarter’s customer success story comes from Dr. Nora Mores, OB/GYN from The Prenatal Diagnoses Center, Luxembourg.
"For me it is worth gold, my pathologies and borderline findings immediately get a second opinion from Prof. Florian Faschingbauer from Erlangen, which helps me a lot." - Dr. Nora Mores, OB/GYN
Read all about how Dr. Nora Mores shares the benefits of Tricefy patient sharing and collaboration. here:
https://academy.triceimaging.com/help/dr-nora-mores-use-case
For more customer use cases, inspiration and “We Use Tricefy” logos, click here:
https://academy.triceimaging.com/help/we-use-tricefy
|
 Copyright © 2016-2018 Trice Imaging, Inc. All rights reserved. 1343 Stratford Court
Del Mar, CA 92014
Copyright © 2016-2018 Trice Imaging, Inc. All rights reserved. 1343 Stratford Court
Del Mar, CA 92014